Silver Knight™ breathing systems include anti-microbial protection designed to help slow down the proliferation of micro-organisms and reduce cross infection within the Intensive Care environment. On heated wire Silver Knight™ breathing systems, both the green inspiratory and the lilac expiratory limbs include the anti-microbial additive for added protection against healthcare acquired infections
Helping in the fight against Hospital Acquired Infections (HAIs)
In the continued fight against Hospital Acquired Infections, Intersurgical has developed the Silver Knight™ range of products with an anti-microbial additive to further enhance patient safety. This anti-microbial, silver-based ion additive has been introduced to a range of breathing systems for both anaesthesia and intensive care use. Testing to ISO Standard 22196 shows a reduction in bacterial viable count by 99.9% against controls2.
How does it work?
Silver Knight™ is an anti-microbial additive that uses silver ions to disrupt the normal enzymatic activities of bacteria. It functions as a safe, quick and effective catalyst to deactivate pathogenic bacteria and prevent their proliferation.
The silver ions bind with the bacteria and damage the cell wall, disrupting normal cellular functions and preventing further proliferation of the bacteria. The silver ions work through catalytic oxidation therefore they are not used up following a single reaction, it may catalyse an innumerable number of reactions. This is one reason for the effectiveness of silver as an anti-microbial agent.
Silver Knight™ is proven¹ to help reduce the incidence of MRSA infection and other organisms including Staphyloccus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter calcoaceticus and Escherichia coli. This technology will reduce microbial growth within, and on, the surface of the breathing systems.
Silver Knight™ breathing systems are all validated for up to seven days use, and remain active, in unopened packaging, for up to five years.
Reference 1: Intersurgical Ltd. Certified Independent Test Results, 2019.
Dräger is a registered trademark of Drägerwerk AG & Co. KGaA. Aquapor is a trademark of Dräger Medical GmbH.
On this page
Adult 22mm breathing systems
- Flextube™ breathing systems for passive humidification
- Smoothbore breathing systems for passive humidification (non-heated wire)
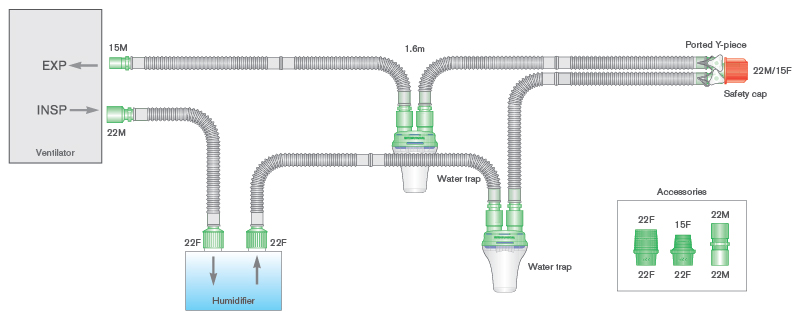
- Smoothbore breathing systems for active humidification with self-sealing water traps
- Dri-Therm™ dual heated wire breathing system
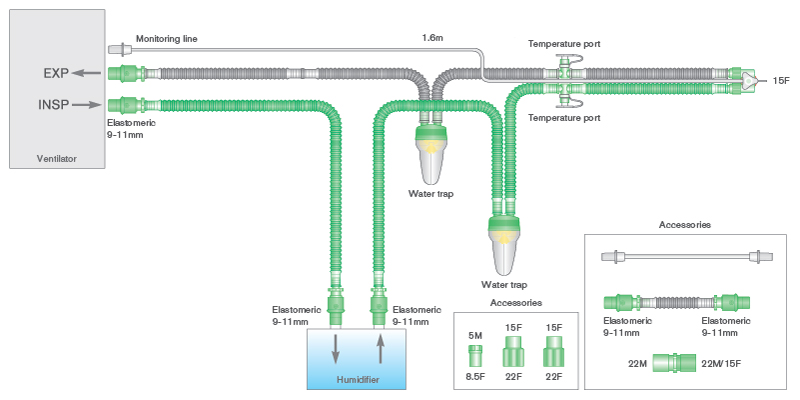
- Flextube™ breathing systems for active humidification with heated wire
- Smoothbore breathing systems for active humidification with heated wire
- Silver Knight™ anti-microbial Flextube™ breathing systems
- Nitric Oxide breathing system and kit