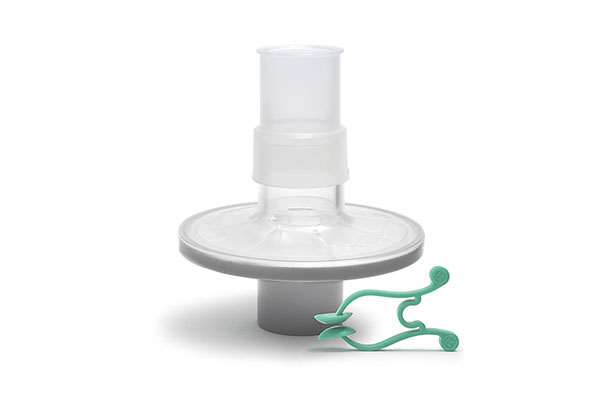
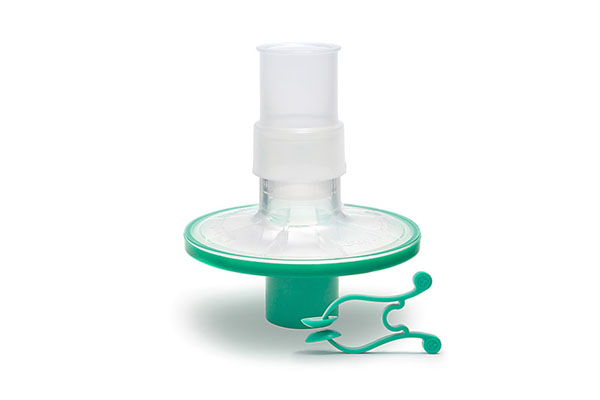
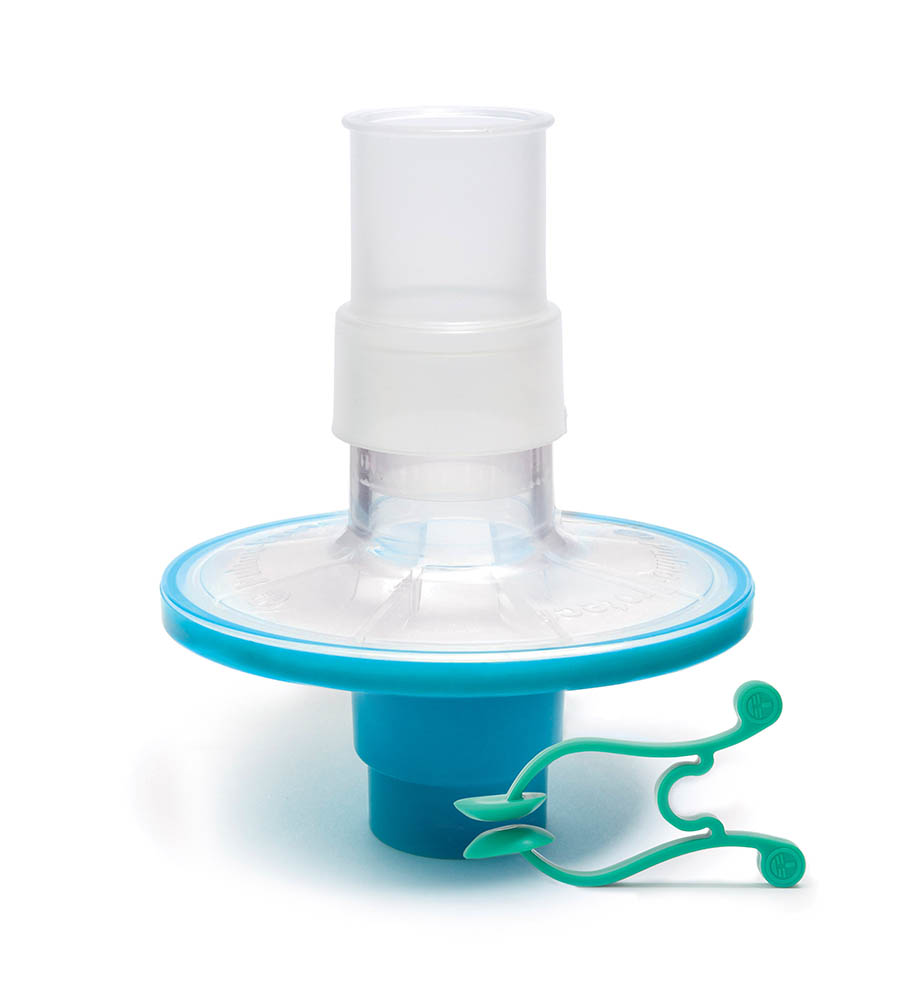
Pulmo-Protect™ provides:
- Protection to pulmonary function test equipment.
- High filtration properties reduced risk of contamination between patients.
- Low resistance performance ensures efficiancy of results and complies with ATS/ERS recommendations1.
- Low functional volume.
- Microbiological filtration effectiveness has been independently tested and validated to provide >99.999% efficiency against bacterial and viruses2
- Flexible, comfortable, disposable mouth piece helps with patient compliance and improve the effectiveness of the test.
- Comfortable single patient nose clip prevents patient-to-patient touch contamination.
- High filtration properties reduces risk of contamination between patients.
- Device compatible range of colour coded filters available individually or as a complete pulmonary function test kit.
Products with the corresponding number are compatible with the following test devices:
1) JAEGER®, MasterScreen, SensorMedics®, Vmax®, Micro Medical, Chest and Microgard®.
2) Medisoft, BodyBox, HypAir Compact + and SpiroAir
3) Fukuda Denshi®, SP-350 and Fudac-77
4) NSpire®
JAEGER is a registered trademark of CareFusion Germany 234 GmbH. SensorMedics is a registered trademark of Sensormedics Corporation. Vmax is a trademark of SensorMedicas Corporation. Microgard is a registered trademark of Becton Dickinson and Company. Fukuda Denshi is a registered trademark of Fukuda Denshi Co. Ltd. KoKo is a trademark of nSpire Health, Inc. NSpire is a trademark of nSpire Health Inc.
References:
1. Journal of Respiratory Medicine 2005.09.015 An audit into the efficacy of single use bacterial/viral filters for the prevention of equipment contamination during lung function assessment.
2. European Respiratory Journal 2005; 26: 319–338 Standardisation of spirometry M.R. Miller, J. Hankinson, V. Brusasco, F. Burgos, et al. 3. Nelson Labs 771942B.1 4. Nelson Labs 771943B.1
On this page
Breathing filters
Explore